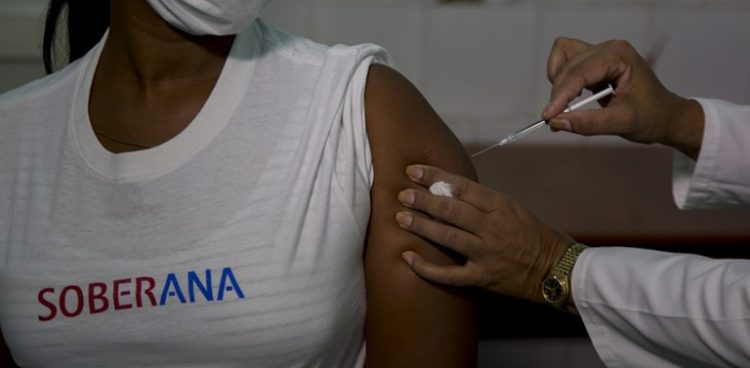
The study uses the Soberana 01 vaccine candidate, the first to receive authorization in Cuba for tests on human beings, and seeks to “stimulate protective levels of neutralizing antibodies against possible reinfection.”
by OnCuba Staff January 26, 2021 in Cuba
Cuba is carrying out a clinical trial in people convalescing from COVID-19, who had a minor clinical picture or were asymptomatic, the official media reported.
The study uses the Soberana 01 vaccine candidate, the first to receive authorization in Cuba for tests on human beings, and seeks to “stimulate protective levels of neutralizing antibodies against possible reinfection,” Prensa Latina (PL) agency reported.
The agency recalled that “globally there are cases of people infected with this coronavirus who were reinfected, even with much more acute and serious forms of the disease,” which is why the trial that is taking place on the island “also seeks to protect this risk group.”
The test began on January 9 at the Institute of Hematology and Immunology and involves 30 volunteers, aged 19 to 59, “who were in contact with the virus and currently have a negative PCR.” These people received a single dose of the vaccine on day 16 and “from that moment on, they are being closely monitored through doctor’s offices and direct contact with the researchers.”
Dr. Rolando Ochoa, a specialist in Immunology at the Finlay Vaccine Institute, explained that the formulation of Soberana 01 used in the test “is simpler and safer, according to the characteristics of the population group under study.”

You really make it appear really easy together with your presentation however I to find this matter to be really one thing which I think I would never understand. It kind of feels too complex and extremely broad for me. I am having a look forward in your next submit, I will try to get the cling of it!
cialis 20 milligram
Buy Drugs
Thanks for your posting on this blog. From my personal experience, periodically softening right up a photograph could possibly provide the digital photographer with a chunk of an artistic flare. Oftentimes however, that soft cloud isn’t exactly what you had in your mind and can often times spoil a normally good photo, especially if you thinking about enlarging the item.
neurontin 600 mg [url=https://gabapentinneurontin.pro/#]neurontin capsule 400 mg[/url] drug neurontin 200 mg
dapoxetine elder – viagra plus soldier cialis with dapoxetine unit
zithromax purchase online: zithromax capsules australia – cost of generic zithromax
amoxicillin 500mg capsules price: amoxicillin cost australia – where can i buy amoxicillin over the counter uk
http://zithromaxa.store/# zithromax 500
Howdy this is kinda of off topic but I was wanting to know if blogs use WYSIWYG editors or if you have to manually code with HTML. I’m starting a blog soon but have no coding expertise so I wanted to get guidance from someone with experience. Any help would be greatly appreciated!|
[url=http://lisinoprilgp.online/]buy cheap lisinopril[/url]
neurontin 200 mg capsules [url=http://gabapentinneurontin.pro/#]neurontin 300 mg mexico[/url] where can i buy neurontin from canada
[url=https://asynthroid.com/]can you buy synthroid in mexico[/url]
This blog post is excellent, probably because of how well the subject was developed. I like some of the comments too.
buy doxycycline: 200 mg doxycycline – doxycycline 100mg price
Thanks for your recommendations on this blog. One particular thing I wish to say is that often purchasing consumer electronics items on the Internet is not new. The fact is, in the past 10 years alone, the market for online electronic products has grown drastically. Today, you could find practically any specific electronic device and gizmo on the Internet, including cameras as well as camcorders to computer spare parts and game playing consoles.
Scam
Hi there, just become alert to your blog through Google, and found that it is really informative. I?m gonna be careful for brussels. I will appreciate in the event you proceed this in future. Many people will be benefited from your writing. Cheers!
I think one of your commercials caused my internet browser to resize, you may well want to put that on your blacklist.
buy doxycycline: buy doxycycline online without prescription – buy doxycycline hyclate 100mg without a rx
Fantastic goods from you, man. I have take into account your stuff previous to and you’re just too wonderful. I really like what you’ve bought here, really like what you are saying and the way in which in which you assert it. You make it entertaining and you continue to take care of to stay it wise. I can’t wait to read far more from you. This is really a great web site.|
If you desire to take much from this paragraph then you have to apply these strategies to your won blog.|
http://amoxila.pro/# buy amoxicillin 500mg canada
amoxicillin no prescipion [url=https://amoxila.pro/#]antibiotic amoxicillin[/url] amoxicillin from canada
I’m not that much of a internet reader to be honest but your sites really nice, keep it up! I’ll go ahead and bookmark your website to come back later. Many thanks
Very neat blog article. Cool.
[url=https://vatrex.online/]buying valtrex in mexico[/url]
In the awesome design of things you’ll secure an A just for effort and hard work. Where exactly you lost me personally ended up being in your facts. You know, they say, the devil is in the details… And it couldn’t be much more accurate here. Having said that, let me inform you what did do the job. Your article (parts of it) is actually rather convincing which is possibly why I am taking the effort in order to opine. I do not really make it a regular habit of doing that. Second, even though I can easily see the jumps in reasoning you make, I am not necessarily sure of exactly how you appear to unite your points that make the final result. For now I will, no doubt yield to your position however trust in the near future you actually link the facts better.
Porn
azithromycin zithromax: zithromax over the counter canada – where can i buy zithromax in canada
The core of your writing while appearing reasonable originally, did not really sit properly with me after some time. Somewhere throughout the sentences you actually were able to make me a believer unfortunately only for a short while. I however have got a problem with your leaps in logic and you would do nicely to fill in those breaks. If you actually can accomplish that, I could undoubtedly end up being fascinated.
http://zithromaxa.store/# zithromax z-pak
how to buy doxycycline online [url=http://doxycyclinea.online/#]where can i get doxycycline[/url] 200 mg doxycycline
neurontin 800 mg tablets: neurontin tablets – neurontin price uk
zithromax prescription in canada: where can i get zithromax over the counter – zithromax 500mg
http://gabapentinneurontin.pro/# neurontin for sale
gabapentin generic: neurontin 800 mg pill – neurontin 800 mg capsules
I found the information you shared very enlightening, thank you! click for more info
[url=https://tadalafi.online/]tadalafil usa[/url]
Thanks so much for the post.Thanks Again. Much obliged.
prednisone without prescription medication [url=http://prednisoned.online/#]prednisone 20[/url] buy prednisone online india
Porn
cenforce online rag – brand viagra pills trunk
Viagra
clonidine online pharmacy
zithromax 250 mg australia: buy cheap generic zithromax – zithromax 500mg price in india
rohypnol pharmacy
sildenafil dosing
[url=http://tadalafilgf.com/]otc cialis usa[/url]
http://zithromaxa.store/# zithromax canadian pharmacy
Pornstar
neurontin tablets uk [url=http://gabapentinneurontin.pro/#]neurontin pills for sale[/url] neurontin 600 mg
With havin so much content and articles do you ever run into any issues of plagorism or copyright infringement? My blog has a lot of completely unique content I’ve either written myself or outsourced but it seems a lot of it is popping it up all over the web without my permission. Do you know any techniques to help stop content from being ripped off? I’d truly appreciate it.
Great blog post. Some tips i would like to make contributions about is that computer memory ought to be purchased when your computer is unable to cope with anything you do by using it. One can add two RAM boards with 1GB each, as an example, but not one of 1GB and one with 2GB. One should look for the manufacturer’s documentation for one’s PC to be certain what type of memory space it can take.
It is appropriate time to make a few plans for the long run and it’s time to be happy. I’ve learn this submit and if I may I desire to counsel you few fascinating things or tips. Perhaps you could write subsequent articles relating to this article. I wish to read more things approximately it!
There are some fascinating closing dates on this article but I don?t know if I see all of them middle to heart. There’s some validity but I will take maintain opinion till I look into it further. Good article , thanks and we wish extra! Added to FeedBurner as well
whoah this weblog is fantastic i like reading your articles. Stay up the good work! You already know, lots of individuals are looking around for this info, you could aid them greatly.
doxycycline mono: cheap doxycycline online – doxycycline 100mg capsules
dapoxetine buy – cialis with dapoxetine sunset cialis with dapoxetine thin
I discovered your blog web site on google and test a couple of of your early posts. Proceed to maintain up the excellent operate. I just further up your RSS feed to my MSN News Reader. In search of ahead to studying extra from you afterward!?